| METHOD | PRINCIPLE OF THE TEST |
|---|---|
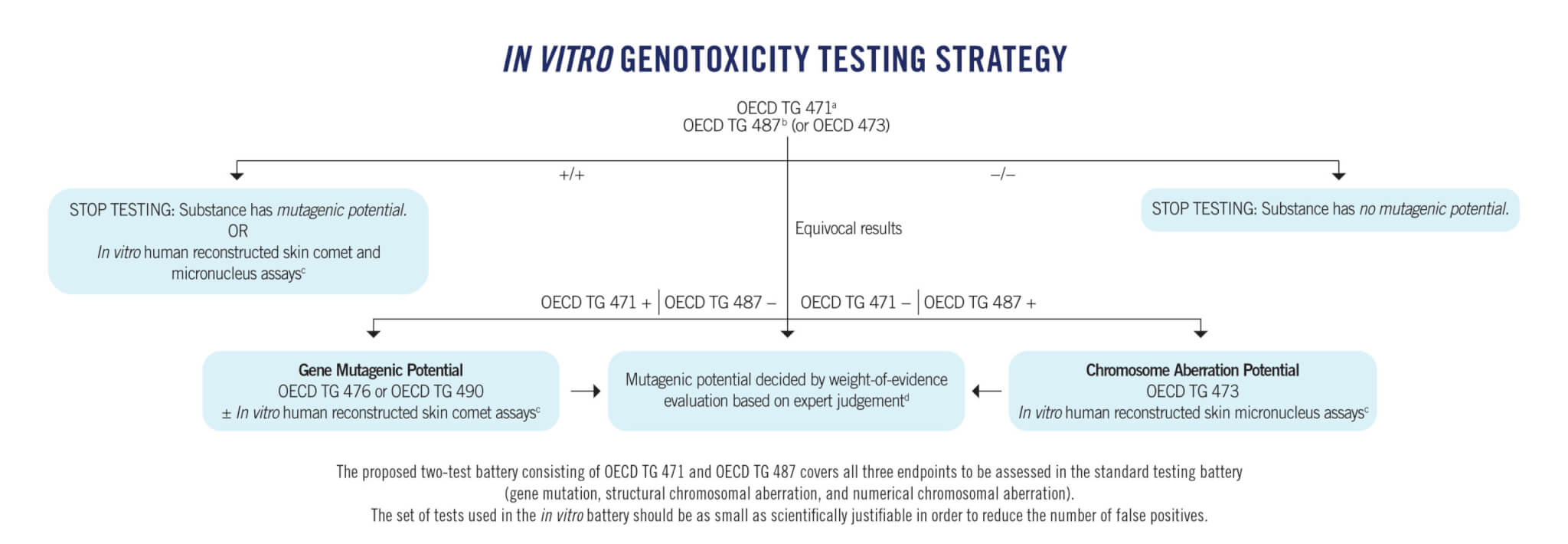
| OECD TG 471: Bacterial Reverse Mutation Test (Ames test) | The Ames test uses at least five strains of amino acid–requiring Salmonella typhimurium and Escherichia coli to detect point mutations by base substitutions or frameshifts. It detects mutations that revert other mutations present in the test strains, restoring the functional capability of the bacteria to synthesise an essential amino acid. A very large database of results for a wide variety of structures is available for bacterial reverse mutation tests, and well-established methodologies have been developed for testing chemicals with different physicochemical properties, including volatile compounds. |
| OECD TG 476: In Vitro Mammalian Cell Gene Mutation Tests Using the HPRT and XPRT Genes | This test measures mutation at hypoxanthine-guanine phosphoribosyltransferase (HPRT) and at a transgene of xanthine-guanine phosphoribosyltransferase (XPRT). The HPRT and XPRT mutation tests detect different spectra of genetic events. Mutant frequency is determined by seeding known numbers of cells in a medium containing the selective agent in order to detect mutant cells and in a medium without the selective agent in order to determine the cloning efficiency (viability). After a suitable incubation time, colonies are counted. |
| OECD TG 487: In Vitro Mammalian Cell Micronucleus Test (MNvit) | The MNvit detects micronuclei in the cytoplasm of interphase cells. Micronuclei may originate in acentric chromosome fragments (i.e. those lacking a centromere) or whole chromosomes that are unable to migrate to the poles during the anaphase stage of cell division. The assay detects the activity of clastogenic and aneugenic test substances in cells that have undergone cell division during or after exposure to the test substance. |
| OECD TG 473: In Vitro Mammalian Chromosomal Aberration Test | This method identifies agents that cause structural chromosomal aberrations in cultured mammalian somatic cells. At predetermined intervals after the exposure of cell cultures to the test substance, the cells are treated with a metaphase-arresting substance, harvested, and stained. Metaphase cells are then analysed microscopically for the presence of chromosomal aberrations. |
| OECD TG 490: In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene | This TG includes two distinct in vitro mammalian gene mutation assays requiring two specific thymidine kinase (TK) heterozygous cell lines: L5178Y tk+/− 3.7.2C cells for the mouse lymphoma assay and TK6 tk+/− cells for the TK6 assay. Genetic events detected using the TK locus include both gene mutations and chromosomal events. Mutant frequency is determined by seeding known numbers of cells in a medium containing the selective agent in order to detect mutant cells and in a medium without the selective agent in order to determine the cloning efficiency (viability). After a suitable incubation time, colonies are counted. |
| In Vitro Human Reconstructed Skin Comet Assay | The comet assay detects the migration of DNA in an agarose gel following electrophoresis, which results in a “head” of intact DNA and a “tail” of fragmented DNA. Single cells are isolated from a highly differentiated three-dimensional tissue model consisting of normal, human-derived epidermal keratinocytes cultured on specially prepared tissue culture inserts and evaluated by fluorescence microscopy after staining with a DNA-binding fluorescent agent. The percentage of DNA in the tail of the comet is used as a measure of DNA damage. |
| In Vitro Human Reconstructed Skin Micronucleus (RSMN) Assay | Like the MNvit, the RSMN test detects micronuclei in the cytoplasm of interphase cells – in this case, a highly differentiated three-dimensional tissue model consisting of normal, human-derived epidermal keratinocytes cultured on specially prepared tissue culture inserts. |
-
Notes
aPotential false positive results can be reduced by investigating the impact of bacteria-specific metabolism.
bPotential false positive results can be reduced by using p53-competent human cells, choosing measures of cytotoxicity based on cell proliferation, and carefully checking the source and the characterisation of the cells.
cTier 2 assays include the in vitro human reconstructed skin micronucleus and comet assays, which are most applicable to testing dermally applied substances. For substances administered by other routes, methods based on 3-D liver or airway models or the HET-MN may be more relevant. (HET-MN uses animal embryos, thus, it should be used only to replace a regulatory requirement for a test on live animals at later life stages.)
dThis may include information on toxicodynamics, toxicogenomics, toxicokinetics, mechanism of toxicity, involvement of reactive oxygen species, saturation of detoxification mechanisms, gene expression profiles, and cell transformation assays. As an example, the European Chemicals Agency has waived tier 2 in vivo studies based on the ToxTracker assay and readacross data.
-
Select Publications
- Groff K, Evans SJ, Doak SH, et al. In vitro and integrated in vivo strategies to reduce animal use in genotoxicity testing. Mutagenesis. 2021;36:389-400.
- Pfuhler S, Downs T, Hewitt N, et al. Validation of the 3D reconstructed human skin micronucleus (RSMN) assay: an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis. 2021;36:1-17.
- Pfuhler S, Pirow R, Downs TR, et al. Validation of the 3D reconstructed human skin comet assay, an animal-free alternative for following-up positive results from standard in vitro genotoxicity assays. Mutagenesis. 2021;36(1):19-35.
- Pfuhler S, van Benthem J, Curren R, et al. Use of in vitro 3D tissue models in genotoxicity testing: strategic fit, validation status and way forward. Report of the working group from the 7th International Workshop on Genotoxicity Testing (IWGT). Mutat Res Genet Toxicol Environ Mutagen. 2020;850-851:503135.
- Conway GE, Shah UK, Llewellyn S, et al. Adaptation of the in vitro micronucleus assay for genotoxicity testing using 3D liver models supporting longer-term exposure durations. Mutagenesis. 2020;35(4):319–330.
- Reisinger K, Dony E, Wolf T, Maul K. Hen’s egg test for micronucleus induction (HET-MN). Methods Mol Biol. 2019;2031:195-208.
- European Commission, Joint Research Centre. Zuang, V, Dura, A, eds. EURL ECVAM Status Report on the Development, Validation and Regulatory Acceptance of Alternative Methods and Approaches (2019). EUR 30100 EN. Publications Office of the European Union; 2020.
- Scientific Committee on Consumer Safety. The SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation 10th Revision. European Commission; 2018.
- Corvi R, Madia F. In vitro genotoxicity testing – can the performance be enhanced? Food Chem Toxicol. 2017;106:600-608.
- Organisation for Economic Co-operation and Development Environment, Health and Safety Division. Overview of the set of OECD Genetic Toxicology Test Guidelines and updates performed in 2014–2015: series on testing and assessment no 238. OECD. Published 22 August 2017. Accessed 24 February 2022. https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2016)33/rev1&doclanguage=en.
- Corvi R, Madia F, Worth A, Whelan M. EURL ECVAM Strategy to Avoid and Reduce Animal Use in Genotoxicity Testing. EUR 26375. Publications Office of the European Union; 2013.
- European Chemicals Agency. N,N,4-trimethylpiperazine-1-ethylamine. European Chemicals Agency. Accessed 24 February 2022. https://echa.europa.eu/nl/registration-dossier/-/registered-dossier/27533/7/7/1.
