Receive a recombinant antibody to replace your animal-derived antibody
PETA Science Consortium International e.V., the Physicians Committee for Responsible Medicine (PCRM), and the Alternatives Research and Development Foundation (ARDF) are offering grants for free catalogue recombinant antibodies for use in research and testing.
Highlighted Antibodies
Flag-tag, HA-tag, and His-tag animal-free recombinant antibodies were successful in immunofluorescence in fixed cells in a recent publication in FEBS Open Bio. Read the publication here and apply to receive the antibodies for free below.
Antibodies from animal sources are one of the largest contributors to the lack of reproducibility of research, leading to the growing use of recombinant antibodies. Since their generation starts from a DNA sequence, recombinant antibodies are consistently reproducible and more reliable than animal-derived monoclonal and polyclonal antibodies.
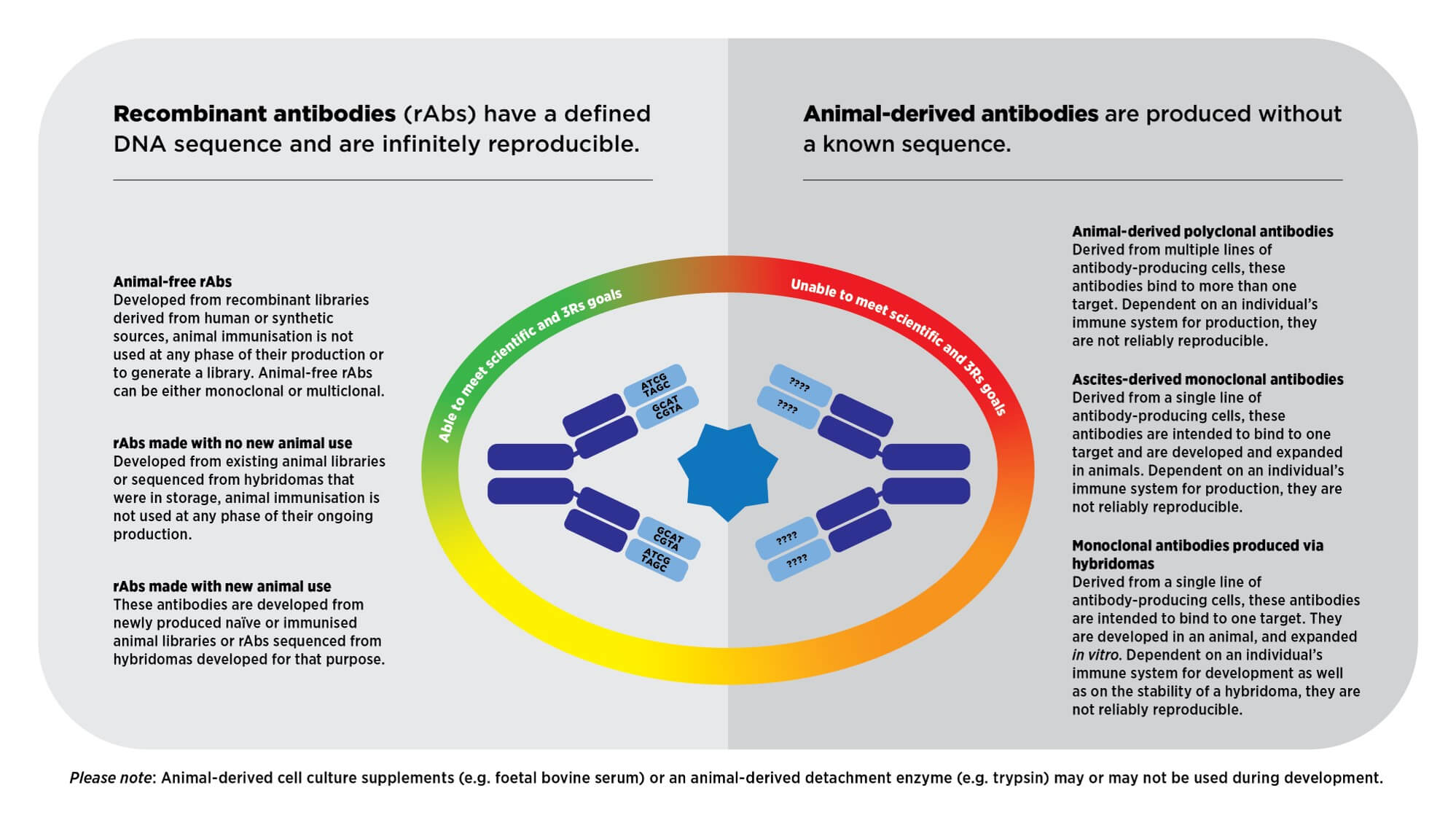
Researchers from any sector (e.g., academia, industry, or government) can apply to receive a recombinant antibody to test in their in vitro applications of interest.
Grant Details
- The animal-derived monoclonal or polyclonal antibody that applicants are interested in replacing must have a catalogue (existing, commercially offered) recombinant antibody available for the same target and research conditions from a facility with experience producing recombinant antibodies, such as those antibodies listed here.* Sequenced recombinant antibodies, such as those available from Absolute Antibody, Cape Biologix, and Abcam, are also eligible. The Science Consortium, PCRM, and ARDF can assist in locating a suitable recombinant antibody, if desired by the applicant.
- Applicants can apply 1) for a recombinant antibody that they have not used previously or 2) for a recombinant antibody that they have previously used but in a different application than what is being proposed.
- Recipients may use the recombinant antibody independently or alongside the currently used animal-derived monoclonal or polyclonal antibody it would replace following testing.
- Recipients must agree to troubleshoot with the Science Consortium, PCRM, and ARDF on any issues with using the recombinant antibody, if needed.
- Recipients must submit the results of testing the recombinant antibody for publication within 2 years.
- Recipients are eligible to receive grants for up to two recombinant antibodies per laboratory.
- Grant amount will vary depending on details of the project. After finalizing the type and amount of antibody, grant recipients will purchase the antibody and provide a receipt or invoice that will be reimbursed by PETA Science Consortium International e.V., PCRM, and ARDF.
* Unsure if you are eligible to apply? Send an email to Katherine Groff at [email protected] to discuss! *
How to Apply
In addition to a curriculum vitae, please submit a proposal (500 words or less) addressing the following:
- The antibody you are interested in replacing with a recombinant antibody and anticipated µl needed
- The objective and status (including progress to date and funding) of your research for which you would be using the antibody
- Your ability and commitment to troubleshoot with the Science Consortium, PCRM, and ARDF on any issues that may arise and publish the results
Within 3 months of receipt of the antibody, recipients shall send the Science Consortium, PCRM, and ARDF an update on their experience using the new antibody.
Must be 18 or older. No purchase necessary. Void where prohibited by law. By applying, you are acknowledging that you have read and agree to PETA Science Consortium International e.V. terms and conditions and privacy policy, as well as PCRM’s privacy policy.
This challenge has a rolling deadline, and recipients will be selected based on their application proposal. For additional information or to apply, please contact Katherine Groff at [email protected].
* Grants are restricted to synthetic and human-derived recombinant antibodies or sequenced antibodies except in the case of replacing polyclonal and ascites-derived monoclonal antibodies.
